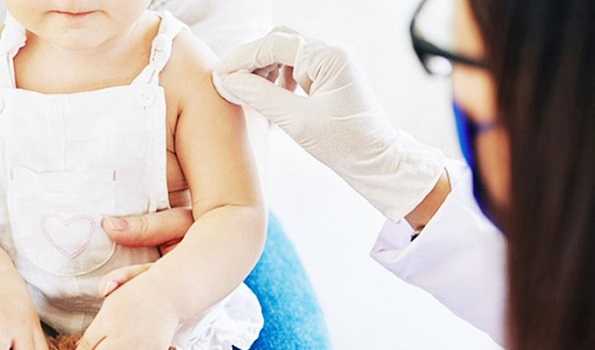
New Delhi : Hyderabad based pharmaceutical firm Biological E Limited has received approval from the Drugs Controller General of India (DCGI) to conduct phase 2/3 clinical trials of its ‘Made in India’ Covid-19 vaccine on children aged between 5 and 18 years with certain conditions, officials said.
The permission was granted on Wednesday night on the recommendation of the Subject Expert Committee (SEC) on Covid-19 drugs of the Central Drugs Standard Control Organisation (CDSCO).
Officials informed that the vaccine, Cobervax, will be tested across ten sites in the country.
The phase 2/3 study will be conducted under the title ‘A Prospective, Randomised, Double-blind, Placebo-controlled, Phase-2/3 Study to Evaluate Safety, Reactogenicity, Tolerability and Immunogenicity of Corbevax Vaccine in Children and Adolescents’, they added.
The government has made an advance payment of Rs 1,500 crore to Biological E for the 30 crore jabs of Cobervax. This would be the fourth vaccine to conduct trials on children in India after Bharat Biotech, Serum Institute and Zydus Cadila.
India has approved only one Covid-19 vaccine for individuals aged less than 18 years. Last month, indigenously developed Zydus Cadila’s needle-free COVID-19 vaccine ZyCoV-D had received Emergency Use Authorisation (EUA) from the drug regulator, making it the first vaccine to be administered in the age group of 12-18 years in the country.
Covaxin from Bharat Biotech is under phase 2/3 clinical trials for the age group of 2 to 18 years. The vaccine is already being authorised to 18 above population under the national Covid-19 immunisation programme.










More Stories
NDA as 15 in Northeast’s 25 seats go to polls: PM
Not Big Fan Impact Player; Its Development of All-Rounders, Says Rohit
Modi will come with hope in 2024: PM