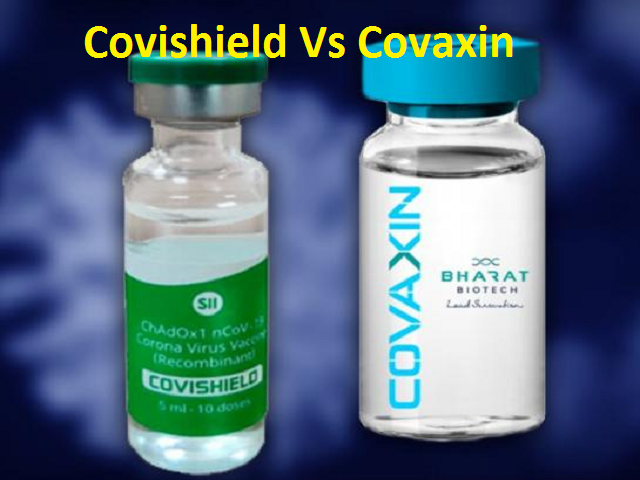
New Delhi: The Subject Expert Committee (SEC) under the Central Drug Regulatory Authority recommended upgrading the status of Covid-19 vaccines Covishield and Covaxin from restricted emergency use to regular marketing authorisation with certain conditions, the Central Drugs Standard and Control Organisation (CDSCO) said on Wednesday.
The central body also said the recommendations will be evaluated by the Drug Controller General of India (DCGI) before the final decision.
“SEC of CDSCO has recommended for upgrade of Covishield and Covaxin status from restricte duse in emergency situations to grant new drug permission with conditions in adult population,”the CDSCO posted on Twitter.
“DCGI will evaluate the recommendations and give its decision,” the central body added to thepost.
The Officials at CDSCO told UNI that the recommendation by SEC has been given with a condition that both the Covid-19 vaccines will be available only at hospitals and clinics registered with the Central government’s Co-WIN portal.
Besides, the recommendation stated that regular authorisation should be given only forad ministration in the adult population, they added.
Bharat Biotech’s Covaxin is also been used in Covid-19 vaccination among the adolescents aged15-17 years.
Both the vaccines were approved in January last year for restricted use in an emergency situation and have been used in the nationwide Covid-19 vaccination drive.









More Stories
LS elections: 4th phase polling records 63 pc turnout
‘Still poor country’: India going to become third largest economy in world
Viksit Bharat, Viksit Odisha: PM Appeals to State People to Vote for BJP